

As the price of electricity from photovoltaics and wind turbines continues to plummet, the prospects of using electrocatalysis to produce fuels and chemicals in a sustainable way become increasingly viable. Current state-of-the-art electrocatalysts are not yet efficient enough to make such catalytic processes economically feasible, thus the design of new electrocatalysts for energy transformation is a major challenge to science. To this end, a fundamental understanding of the atomic-scale processes that dictate reactivity is essential for progress toward a sustainable energy future.
CSTCC 2022 Photobook
CSTCC 2024 Photobook
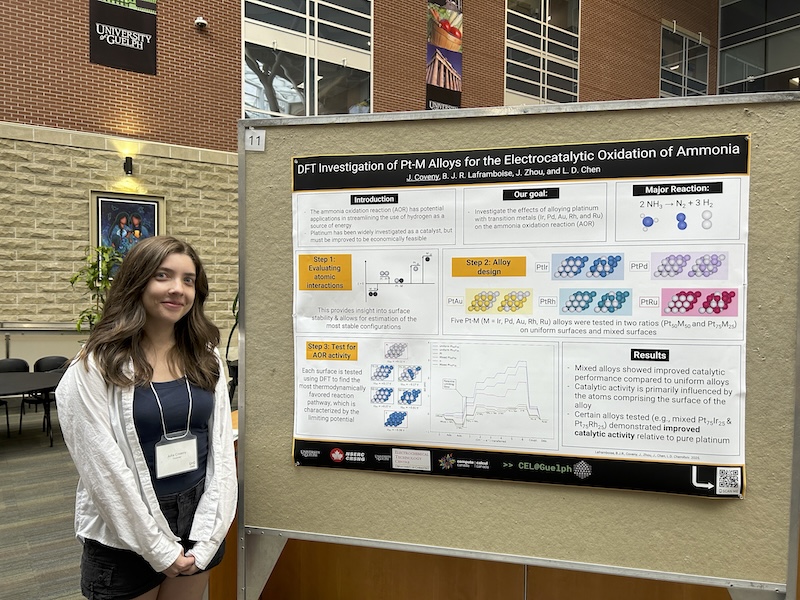
Research in the Computational Electrochemistry Laboratory (“CEL@Guelph”) is led by Prof. Leanne D. Chen and focuses on using ab initio computational methods to model reactions occurring at the electrode-electrolyte double-layer—arguably the most important component in an electrochemical cell. Gaining atomic-scale insight at this interface would have immense impact on the rational design of fuel cells, batteries, and other energy transformation systems such as the electroreduction of carbon dioxide. The following presentations provide more information about research in CEL@Guelph:
ETC-ECS Student Chapter Speaker Series 1
Celebrating the Life of Suning Wang at CCCE 2021
Symposium in Honour of Jens Nørskov’s 70th Birthday
Third Annual ECS Guelph Young Researcher Symposium
If you are a curiosity- and teamwork-driven undergraduate student looking to
carry out a 4th year project or explore possibilities for a graduate degree,
please do not hesitate to send Prof. Chen an email.
CSTCC 2022 Photobook
CSTCC 2024 Photobook
Research in the Computational Electrochemistry Laboratory (“CEL@Guelph”) is led by Prof. Leanne D. Chen and focuses on using ab initio computational methods to model reactions occurring at the electrode-electrolyte double-layer—arguably the most important component in an electrochemical cell. Gaining atomic-scale insight at this interface would have immense impact on the rational design of fuel cells, batteries, and other energy transformation systems such as the electroreduction of carbon dioxide. The following presentations provide more information about research in CEL@Guelph:
ETC-ECS Student Chapter Speaker Series 1
Celebrating the Life of Suning Wang at CCCE 2021
Symposium in Honour of Jens Nørskov’s 70th Birthday
Third Annual ECS Guelph Young Researcher Symposium
If you are a curiosity- and teamwork-driven undergraduate student looking to
carry out a 4th year project or explore possibilities for a graduate degree,
please do not hesitate to send Prof. Chen an email.
Research Keywords
Catalysis
Electrochemistry
Materials & Interfaces
Density Functional Theory
Molecular Dynamics
Microkinetic Modelling
Just Announced
Recent Success
Congratulations to Leanne for receiving a Research Excellence Award!
Recent Success
Congratulations to Jingwen for receiving the Jacek Lipkowski Award!